Tuberculosis (TB): an infectious disease whose name has become very familiar amongst South Africans and the rest of the world. When it comes to the global burden of TB, South Africa ranks third on the list, with the disease being the country’s leading cause of death. Looking at the statistics, some of us might be left quite astonished: According to the World Health Organization (WHO), it is estimated that approximately 322,000 people became infected with TB in 2017 alone, with 56,000 South Africans dying as a result of the disease.
TB is caused by a type of bacteria/germ known as Mycobacterium tuberculosis (M.tb), which most commonly infects the lungs. Think of M.tb as the ‘magician’ of the bacterial world. It has many tricks up its sleeve; for example, it has found ways to disappear from the body’s surveillance systems and has mastered the art of escaping the ‘death trap’ through the manipulation of the host’s molecular pathways. With that being said, much like a good magician, M.tb is reluctant to reveal its tricks and scientists involved in TB research are left as the puzzled spectators trying to unravel the mysteries behind the “magic”.
A small research team at Stellenbosch University have dedicated their time and effort into uncovering the secrets of this bacteria, with hopes of coming to a better understanding of how the bacteria works and which molecules are important for its survival. In so doing, it opens up the potential for the development of new and more effective TB treatments.
M.tb and Autophagy: The great escape
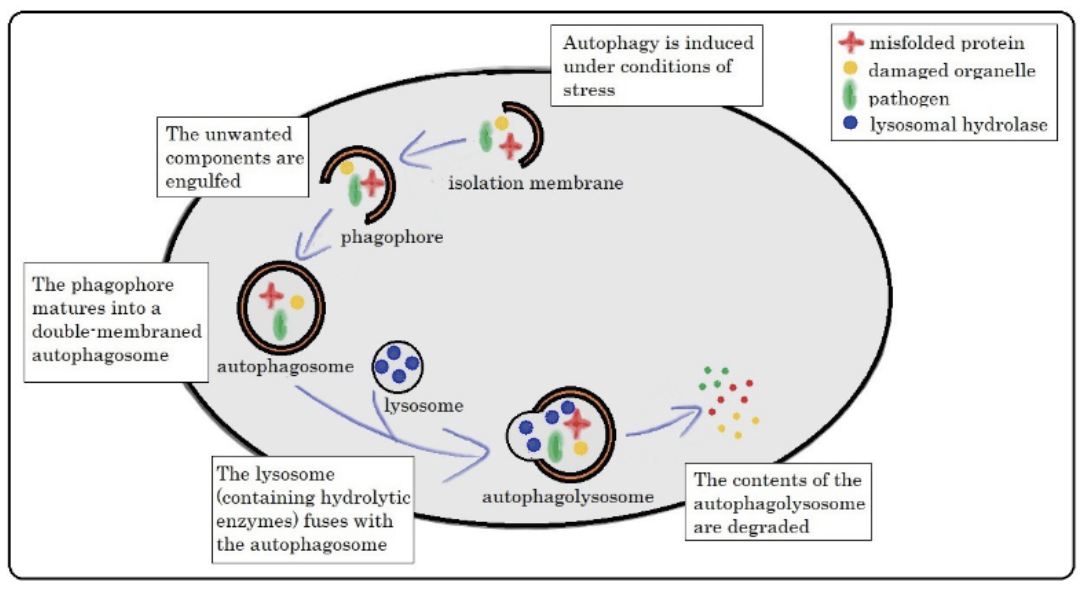
In order to remain healthy, every cell in our body is maintained in a state of balance. This means that any unwanted or foreign material needs to be broken down and removed. This might include old proteins, damaged or dysfunctional parts of the cell, as well as invading micro-organisms. One method that is used to accomplish this is Autophagy (see figure 1), a process whose name literally translates to “self-eating”. When autophagy is activated, the unwanted components become encapsulated into a double-membraned vesicle (autophagosome). This vesicle then fuses together with an acidic lysosome to form an autophagolysosome. The lysosome contains many degradative enzymes that function to destroy the unwanted material, but it is important to note that they will only work properly when in an acidic environment (pH 4.5 – 5.0). Anything that prevents this environment from becoming acidic will disrupt the autophagy process and as a result, the unwanted components won’t be degraded.
When M.tb infects someone, the first defence cells to arrive at the scene are known as macrophages. Macrophages are white blood cells that are capable of locating and destroying foreign particles, for example viruses and bacteria. Autophagy is one of the ways in which macrophages kill these invaders and in the past decade, its importance in defence against M.tb infection has become evident. However, there is a catch: over the years, M.tb has found ways to avoid being killed by autophagy and therefore, live and grow inside macrophages. It does this by either stopping the fusion of the lysosome with the autophagosome or by preventing the acidification of the autophagolysosome, but exactly how it achieves this is still fairly unknown.
Where does Snapin fit in?
The Snapin protein is commonly known to function in the brain, but has recently been shown to have a function in macrophages. Shi and colleagues showed that Snapin is important for autophagy because it helps maintain the acidic environment of the lysosome. This means that Snapin is needed in order for the degradative enzymes to function properly. We know that macrophages are important for the immune response against M.tb and that autophagy is important for controlling and removing M.tb. However, we do not know where Snapin fits into the equation, as it hasn’t been investigated in the context of bacterial infection. Preliminary findings suggest that it is important for M.tb’s survival within macrophages, as less bacteria survive inside the cell when there are low levels of the protein. It is thought that Snapin might play a role in M.tb’s ability to prevent autophagolysosomal acidification, but whether this is true or not still requires further investigation.
A large amount of research has been done regarding M.tb and autophagy, but scientists have only revealed the tip of the ice berg. By identifying the molecules involved in the immune response against M.tb, as well as those that might be important for M.tb’s survival, scientists can look at new and innovative ways to exploit these molecules in order to help treat and combat TB, and Snapin might just be the missing puzzle piece.