BENEFIT Kids
Dedicated to Advancing MDR-TB Treatment and Prevention for Children
Children with Multidrug-Resistant Tuberculosis (MDR-TB) are a neglected population suffering from a largely neglected disease. Each year, around 30,000 children contract MDR-TB, a highly challenging form of tuberculosis that does not respond to two key anti-TB drugs, isoniazid and rifampicin. Treatment for MDR-TB is longer, more complicated, and less effective than for drug-sensitive TB — especially for children. The Better Evidence and Formulations for Improved MDR-TB Treatment for Children (BENEFIT Kids) project is a collaborative effort dedicated to researching and accelerating the adoption of innovative MDR-TB treatment regimens and prevention methods for children of all ages, in order to reduce illness and death among this vulnerable population.
Research project bridges treatment gap for children with MDR-T
THE PROBLEM
The treatment journey for children with MDR-TB and their caregivers is long and harrowing. In many places in the world, treatment involves painful daily injections, or taking handfuls of foul-tasting tablets of older, less effective medicines. Treatment can last anywhere from nine months to two years, and may still involve long-term hospitalisation.
Most of the drugs currently given to children with MDR-TB:
![]() Were developed 50 to 60 years ago
Were developed 50 to 60 years ago
![]() Are less effective than first-line treatments
Are less effective than first-line treatments
![]() Have unpleasant side effects — including severe nausea and vomiting
Have unpleasant side effects — including severe nausea and vomiting
Although adults are benefitting from treatment advances, children are not. Key evidence and product gaps — as well as lack of economic incentive for pharmaceutical companies — jeopardises the potential of new innovations to truly transform paediatric MDR-TB treatments.
THE SOLUTION
Children need access to MDR-TB treatment that is effective, safe, and palatable.
When diagnosed and properly treated, children with MDR-TB can be cured and lead healthy lives. But children are not simply “little adults” — and developing drug formulations for children poses a unique set of challenges. Supported by funding from Unitaid, the BENEFIT Kids project is bridging knowledge gaps and producing critical evidence on dosing, safety, and efficacy — evidence that is shaping WHO policy recommendations and facilitating the production of new TB medications tailored for children.
The project is actively engaged in seven clinical treatment and prevention trials across South Africa, the Philippines, and India. Additionally, it is conducting two comprehensive systematic reviews on MDR-TB treatment evidence. These efforts will strengthen the evidence on optimal dosing, safety, efficacy, acceptability and costs of medications for the treatment and prevention of MDR-TB in children, generate data with more child-friendly formulations for MDR-TB treatment and preventive therapies that taste better and establish the world’s first clinical trial on preventing MDR-TB in children.
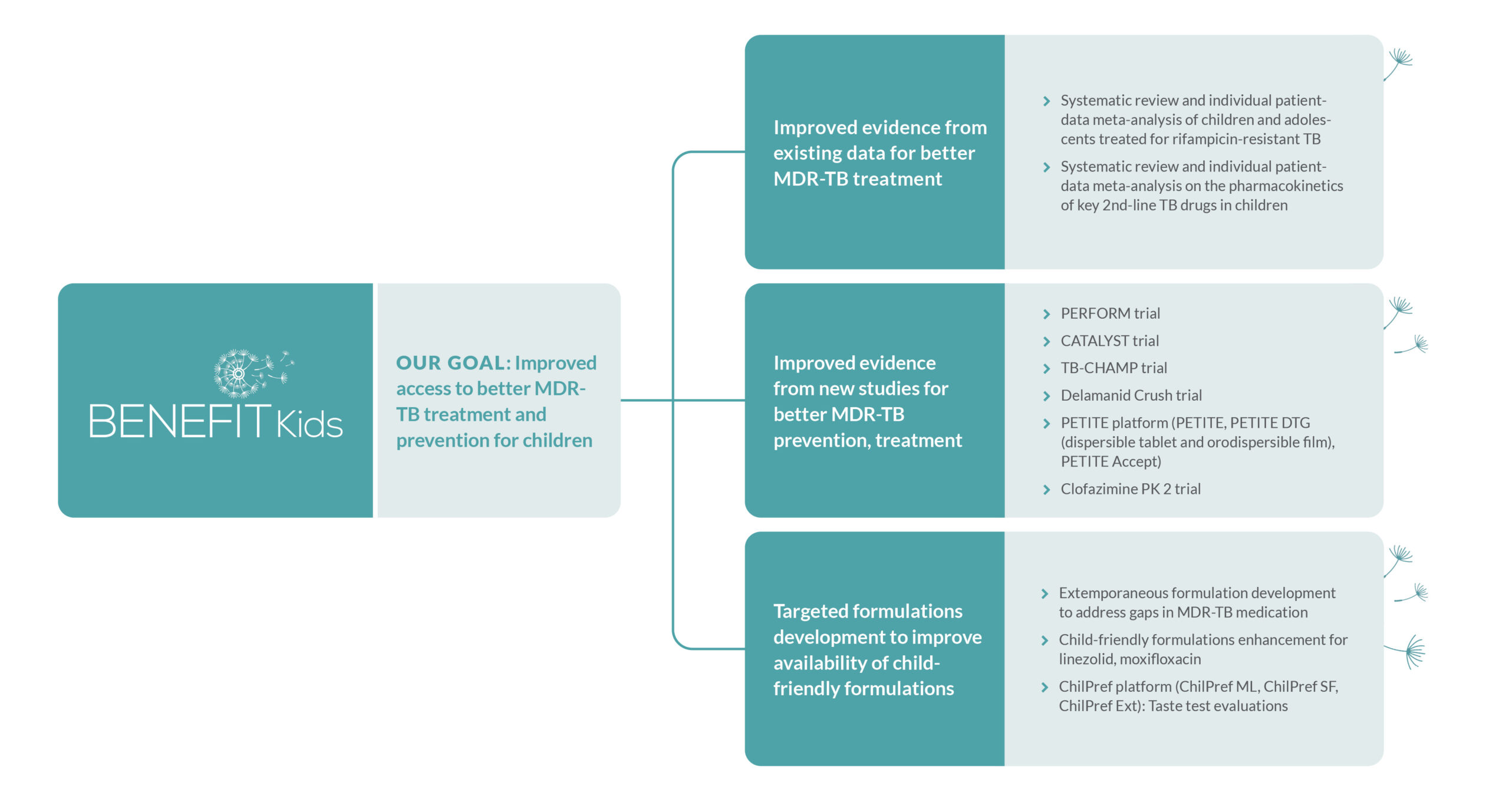

Learn more about the work of the BENEFIT Kids project here:
BENEFIT Kids Partners

Contact Us
- Lario Viljoen : lario@sun.ac.za
- Tina Sachs: tsachs@sun.ac.za



