Clinical Trials Unit


NIH-funded DAIDS Clinical Trials Unit at Stellenbosch University
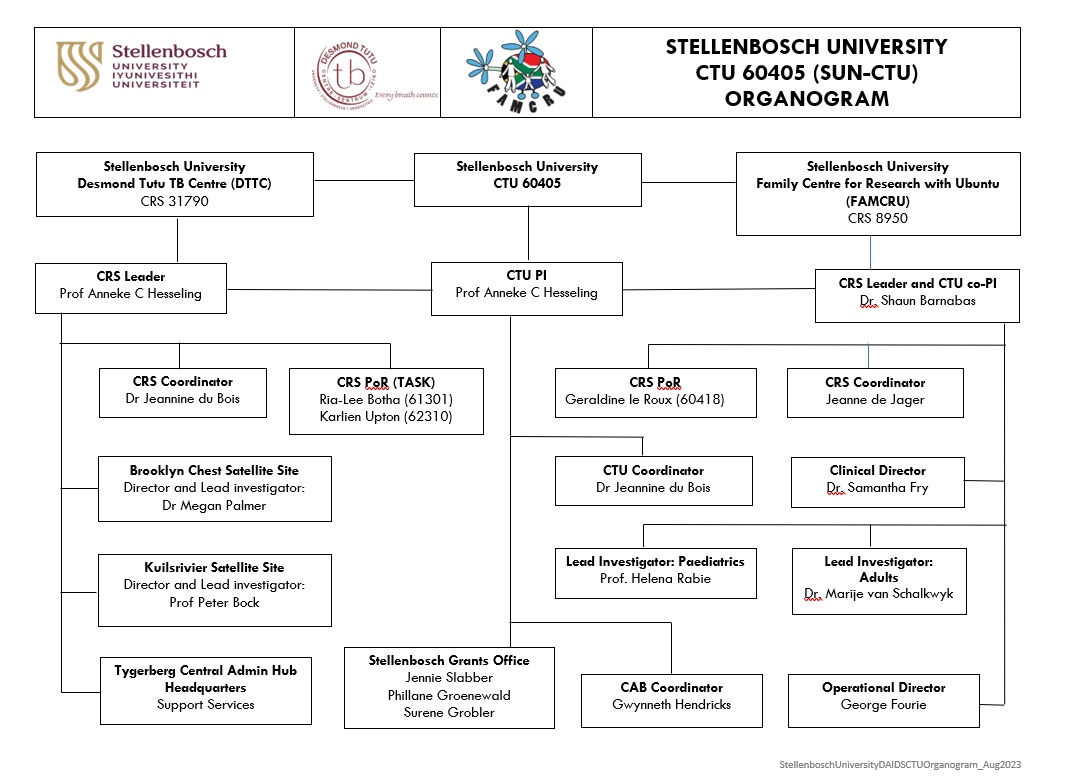
Stellenbosch University is supported through a NIH Uo1 grant to participate in NIH-funded clinical trial network activities through the Division of AIDS (DAIDS). The Stellenbosch University Clinical Trials Unit (SUN-CTU, 60405) is an established, pluripotent, high-capacity research partnership between the Family Centre for Research with Ubuntu (FAMCRU CRS 8950), and the Desmond Tutu TB Centre (DTTC. CRS 31790) which are ideally aligned for participation in DAIDS-funded network studies. The SUN-CTU is jointly led by Anneke Hesseling (PI), and Shaun Barnabas (co-PI)
Our context
South Africa currently has the world’s largest population living with HIV and the second highest tuberculosis (TB) incidence globally, with TB being the leading cause of death from an infectious agent. South Africa is amongst the top 10 countries with high burden of HIV, TB and drug-resistant TB.
Location
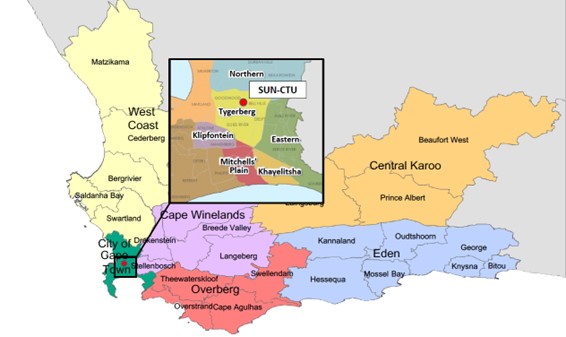
The well-established Stellenbosch University Clinical Trials Unit (SUN-CTU) is centrally located at the Tygerberg campus of Stellenbosch University’s (SU) Faculty of Medicine and Health Sciences (FMHS) in urban Cape Town, Western Cape Province, South Africa (SA). Cape Town, an epicentre of the HIV and TB pandemics, has both high burden of disease and excellent infrastructure for clinical research. SUN-CTU serves a population of approximately 4 million people in the Cape Town Metropolitan area which has a population density of 45 persons per km2, the largest age group being young adults between 25-29 years, accounting for 10.7% of the population. Children < 5 years contribute 9.9%, approximately 396,000 people.
Our vision
The SUN-CTU vision is “a generation free of HIV and tuberculosis.” The SUN-CTU is hallmarked by strong collaboration with local health services, including HIV and TB programs, strong partnerships and equitable engagement with local communities affected by HIV and TB. Our CTU has a strong family-and community-centred approach, focusing on HIV and TB-related and other relevant research in all at-risk populations, spanning low birth weight (LBW) or premature neonates, infants, children, adolescents and young people, pregnant and postpartum women and adults of all ages.
Our objectives
We aim to design and implement high-impact clinical research that will result in safe, patient- and-family-centred, effective prevention, treatment and remission of HIV and its related comorbidities, and TB prevention, diagnosis, and treatment, across all age groups.
The well-integrated and established SUN-CTU is a pluripotent network at the 1) Family Centre for Research with Ubuntu (FAMCRU), for IMPAACT network activities and selected ACTG and CoVPN studies, and 2) the Desmond Tutu TB Centre (DTTC), for IMPAACT and selected HPTN network activities.
Key research strengths and competencies
Across our CTU, we have consistently strived to address the needs of families affected by HIV and/or TB. The SUN-CTU has an excellent track record of implementing trials, capacity building for clinical research and support of routine clinical service provision, evidenced by its highly productive mentored investigator program, active participation in the networks’ scientific agenda, and extensive training programmes with local health services and implementing partners. In addition to its track record of clinical and biomedical research, the SUN-CTU has significant scientific capacity to design and conduct rigorous socio-behavioural research and strongly endorses an approach which integrates biomedical, structural and behavioural work, promoting uptake and adherence of study interventions. Our strong partnership with key local stakeholders, including implementing partners and communities, allows for successful trial enrolment and effective dissemination and uptake of our findings to ensure impact where most needed.
The SUN-CTU also has core competencies in clinical trial design, biostatistics, clinical pharmacology and pharmacometrics, clinical virology, vaccinology, HIV immunology, HIV virology, HIV and neurodevelopment, HIV and noncommunicable diseases, TB microbiology, bio-informatics, TB molecular biology, neurosciences, TB immunology and TB biomarker research, TB in special populations such as the critically ill, post-TB lung and brain disease, implementation science research including the use of large routine datasets, epidemiology, mathematical modelling, infection control prevention, nuclear medicine and advanced imaging modalities.
Underpinning its therapeutic HIV and TB research, our CTU has a strong track record of close collaboration with the International Pharmacology Specialty Laboratory at the University of Cape Town (UCT) and with established trial support laboratories for safety and microbiology evaluation. SUN-CTU has several highly specialized research labs and is building critically needed research capacity for clinical pharmacology of HIV and TB at the Division of Clinical Pharmacology, SU.
SUN-CTU Clinical Research Sites (CRS)
The Desmond Tutu TB Centre (DTTC), CRS 31790 (affiliated with IMPAACT and currently an HPTN protocol-specific site), is an academic research center in the Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences (FMHS), Stellenbosch University. The DTTC was founded in 2004.
The DTTC is a global leader in paediatric TB research and was awarded the Stop TB Partnership Kochon Prize for ground-breaking research on pediatric TB in 2013. The DTTC also received the HPTN Network Staff Excellence in Leadership Award in 2019. The DTTC Director is Anneke Hesseling (MD, PhD). She is the DTTC CRS leader, IMPAACT network lead and CTU Principal Investigator (PI). The HPTN network lead at DTTC is Peter Bock (MD, PhD), Associate Professor, Paediatrics and Child Health at SU. Trials are implemented at the Brooklyn Chest Paediatric Pharmacokinetic trial unit (Medical director: Dr. Megan Palmer) and the Kuilsrivier unit (Medical director: Prof. Peter Bock).
The DTTC has strong competences in TB treatment and prevention research, in drug-resistant and drug-susceptible TB paediatric TB diagnostic research, socio-behavioural research, HIV prevention, treatment and community-based trials, hallmarked by close collaboration with affected communities and healthcare services and partners .
DTTC is based at the central administrative hub at the Tygerberg Medical campus, faculty of Medicine and Health Sciences with key clinical research sites at Brooklyn Chest Hospital and Kuilsrivier.
The DTTC CRS leader is Anneke C. Hesseling (MD, PhD). She is Distinguished Professor in Paediatrics and Child Health at SU and holds a Research Chair National Research Foundation Chair in Pediatric TB. She is scientific chair for the IMPAACT Tuberculosis Scientific Committee (TBSC). Her leadership team includes Peter Bock (MD, PhD, family physician) as HPTN lead investigator, Kuilsrivier HPTN site, Megan Palmer (MD) Medical Director at the Brooklyn Chest Hospital (BCH) Pharmacokinetic (PK) Unit, Jeannine du Bois, the DTTC CRS and the overall SUN-CTU coordinator. Jennifer Hughes (MDR-TB specialist and IMPAACT investigator), Jana Winckler (MD, MPhil Pharmacology, IMPAACT investigator), Louvina van der Laan (MD, PhD), Simon Schaaf (MD, PhD, infectious diseases paediatrician and IMPAACT investigator), Graeme Hoddinott (PhD), lead sociobehavorial scientist, and Anne-Marie Demers (MD), infectious diseases specialist and microbiologist.
DTTC participates in two major focus areas, aligning with our CTU specific aims and IMPAACT and HPTN network research priorities: Tuberculosis in special populations through IMPAACT: The DTTC has key competencies in high-impact paediatric translational TB clinical research, including the prevention, diagnosis and treatment of children across the age continuum and along the spectrum of TB disease, including drug-susceptible (DS-TB) and drug-resistant TB (DR-TB).The DTTC, specifically, has an excellent track record of therapeutic trials in children and pregnant women, including the design, implementation and analyses of phase I, II and III trials of novel and repurposed drugs and regimens for TB treatment and prevention. The paediatric TB research agenda at DTTC is closely aligned with the IMPAACT TBSC agenda, including TB treatment, diagnostics and vaccines in children including neonates, infants and adolescents, and pregnant women, based on stated TB priorities for the IMPAACT network. The scientific focus of DTTC will continue to be evaluation of novel anti-TB treatments, prevention and improving the diagnosis in children and pregnant women affected by DR and DS-TB. We will also focus on novel TB diagnostics, advanced imaging, post-TB lung health, and highly specialized site of disease PK studies in children (e.g. TB meningitis, lymph node disease). We have excellent clinical pharmacology and pharmacometrics expertise in our CRS and CTU (Eric Decloedt, Ahmed Abdulfathi, Jana Winckler, Louvina van der Laan).
HIV prevention and the HPTN: DTTC has extensive experience implementing HIV prevention studies. In future we will continue to focus on HIV prevention studies, in both HIV+ (treatment as prevention) and HIV- individuals (PrEP). DTTC was one of 2 sites for HPTN 071, the largest HIV prevention trial ever completed, evaluating the effect of a combined HIV prevention package on population-level HIV incidence and is one of 20 African sites implementing HPTN 084, an FDA-regulated trial, evaluating intramuscular cabotegravir for PrEP. DTTC utilizes a client and family-centred approach that integrates biomedical, structural and behavioural interventions.
The Family Centre for Research with Ubuntu (FAMCRU), CRS 8950 is affiliated with the IMPAACT network and is a CoVPN and ACTG protocol specific site. It is an academic research centre in the Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University (SU). FAMCRU was established in 2002 by Emeritus Professor Mark Cotton.
The primary research site is located within the associated Tygerberg Academic Hospital (TBH) complex, adjacent to the Family Infectious Diseases Clinic (IDC). There are additional research sites:
- Peri-urban, next to primary health care facilities:
- Michael Mapongwana
- Kraaifontein
- Rural, in the Breede Valley district, approximately 100km away:
- Worcester
FAMCRU focuses on:
- HIV remission/cure
- HIV related non-communicable diseases
- HIV neurocognitive impairment
- Antiretroviral treatment (ART) adherence monitoring and support
- Novel HIV treatment modalities
- New prevention of transmission (PMTCT) regimens
- Management of resistant HIV
- Pharmacokinetic measurement of new ART and TB drugs (all age ranges including preterm or LBW infants and pregnant or postpartum women)
- TB vaccines (including BCG)
- TB prevention and/or TB treatment during pregnancy
The FAMCRU CRS leader, Shaun Barnabas (MD, PhD), is an infectious diseases paediatrician. His leadership team includes senior clinical members with extensive experience in IMPAACT, ACTG, other research networks and industry sponsored trials.
- Lead investigators for IMPAACT Paediatric studies:
- Prof Helena Rabie (MD, PhD, infectious diseases paediatrician)
- Samantha Fry (MD, paediatrician)
- Lead investigator for IMPAACT Maternal and ACTG Adult studies:
- Marije Van Schalkwyk (MD)
- Neonatal studies
- Prof Adrie Bekker (MD, PhD, neonatologist)
- Neurodevelopmental studies
- Prof Barbara Laughton (MD, PhD, neurodevelopmental paediatrician)
- Mentored IMPAACT investigators:
- Lindie Rossouw (MD) and Samantha du Toit (MD)
Jeanne De Jager is the FAMCRU CRS Coordinator and Geraldine Le Roux is FAMCRU Pharmacist of Record. Emeritus Prof Mark Cotton (MD, PhD) continues to provide support and mentorship to the team.
FAMCRU’s focus on cure/remission work has results in the involvement in three pivotal IMPAACT studies. IMPAACT 2028 is an observational study to characterize a cohort of children who may participate in future research related to HIV remission or cure, is chaired by Shaun Barnabas and Samantha Fry. IMPAACT 2039, a protocol in development is a randomized study using a combination HIV vaccination and broadly neutralizing antibodies to study remission and is chaired by Mark Cotton. IMPAACT 1115 explores the effects of early intensive antiretroviral therapy on achieving HIV remission in neonates; Mark Cotton is the vice-chair.
FAMCRU is also involved in the evaluation of novel ART in special populations: in adolescents with IMPAACT 2017 which evaluates cabotegravir and rilpivirine, in pregnancy and postpartum women with IMPAACT 2026 that studies pharmacokinetics of TB and ARV medications and in infants during the first 4-6 weeks of life with IMPAACT 2023 that evaluates dolutegravir.
Cross-cutting socio-behavioural research: Across all research priority areas, our TB and HIV research has been supported by rigorous socio-behavioural research. This will focus on improved understanding of patient experiences of living with TB and/or HIV, prevention and treatment preferences, and the acceptability of therapeutic interventions. We will expand on our well-established capacity for high-quality, multi-method socio-behavioural science through nested work in network protocols. Focus areas have also included stigma, co-morbid mental health challenges in HPTN 071 (PopART) and assessing adherence and retention for both TB and HIV prevention and treatment trials. The novel method of designing qualitative cohort studies has been pioneered at DTTC by Graeme Hoddinott, HPN 071 lead social scientist. Qualitative research, including formative research, has helped identify ideal recruitment strategies and improve understanding of patient experiences across multiple networks, to support adherence and retention. Qualitative research is also linked to CAB consultation. Staff from the socio-behavioural research team at DTTC are frequently co-funded as students.