BENEFIT Kids | Conferences
2023 Union World Conference
The BENEFIT Kids Project, funded by Unitaid, aims to improve MDR-TB treatment for children, improving their health and reducing childhood deaths. We hosted a satellite session at the 53rd Union World Conference on Lung Health 2023 (November 15-18).
Click on the link below to read about the BENEFIT Kids Satellite Session
BENEFIT Kids Satellite Session

Important findings from the BENEFIT Kids Project had also been presented:
- The socio-economic impact of the COVID-19 pandemic in households having children with Rifampicin Resistant TB: Qualitative study from South Africa, India and Philippines.
- Stigma in households of children with rifampicin-resistant tuberculosis in South Africa, India, and the Philippines.
- Palatability preferences in children; the swish-and-spit taste panel approach for formulation development.
- Pharmacokinetic-Toxicity Analysis of Long-term Linezolid Use in Children with MDR-TB.
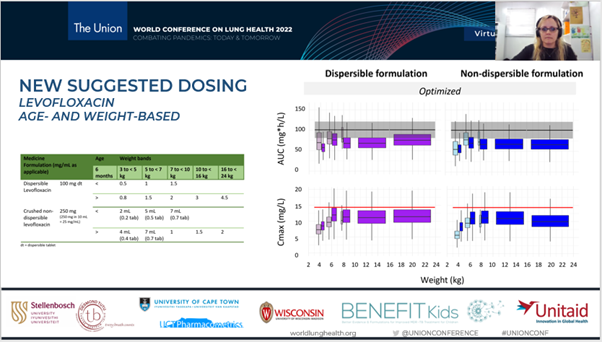
- Pharmacokinetics and optimal dosing of levofloxacin in children: An individual participant data meta-analysis.

Click on the link below to access the BENEFIT Kids Post-Conference Toolkit
2022 Union World Conference
In 2022, we presented important findings at the virtual Union World Conference on Lung Health 2022 (November 8-11) that were well-received:
- Delamanid Tablets for Children: Dispersed 50mg delamanid tablets have the same effectiveness as whole tablets. This means they can be used in children and patients who can't swallow whole tablets, improving treatment accessibility.
- Treatment Outcomes in Young Children: We conducted a global analysis of individual patient data to assess the impact of bedaquiline and delamanid on treatment outcomes in children under 3-6 years old with rifampicin-resistant tuberculosis. This helps us understand their effectiveness and safety in young children.
- Predictors of Treatment Outcomes in Children: In our efforts to enhance the treatment of paediatric rifampicin-resistant tuberculosis (RR-TB), we conducted a comprehensive global review and individual patient data meta-analysis. This extensive study examined demographic, clinical, and treatment factors that influenced outcomes in 20,395 children and adolescents (0-19 years) receiving treatment for RR-TB.
- Optimising Levofloxacin Formulations for Children: We conducted a crossover study comparing the pharmacokinetics of novel dispersible (paediatric) and standard non-dispersible (adult) levofloxacin formulations in children receiving routine preventive therapy for rifampicin-resistant tuberculosis. The study revealed differences in bioavailability and exposure between the formulations, prompting the development of new, optimised dosing strategies based on age and weight for globally available formulations.