Modified Voice Risk Score (MVRS) is a risk assessment tool that is used to determine an individual’s risk of acquiring HIV. The MVRS tool uses 5 standardized questions and assigns a score to each response. The scores are specific to the questions and range from 0 to 2 based on a participant’s response. The scores of each questions are added to give an overall score out of a maximum total score of 8. The scale is then used to determine an individuals risk with 0 meaning low or no risk whilst a score of 8 is high risk. The study requires us to identify individuals with a MVRS ≥5 and our study recruitment team have managed to identify 30% of our enrolled participants who have a MVRS of 8. This in turns means that the HPTN 084 team in Kuilsriver, Cape Town are targeting the correct population to answer the research question.
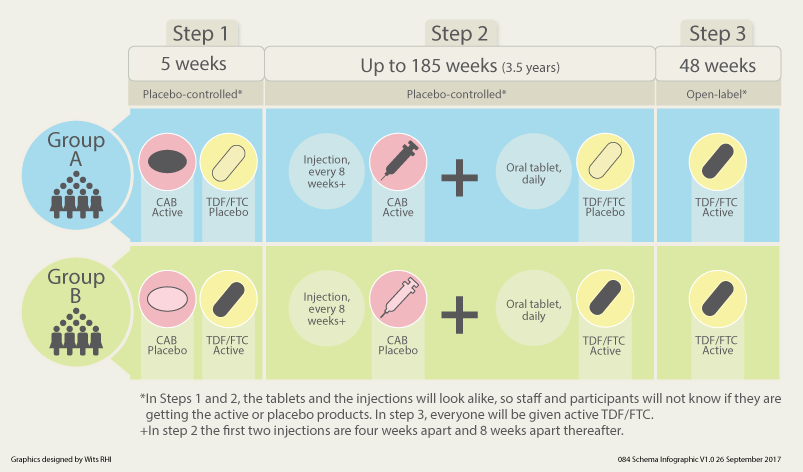
HPTN 084 (The LIFE Study) is a study being done to evaluate the safety and efficacy of the injectable agent, cabotegravir (CAB LA) compared to daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), for pre-exposure prophylaxis (PrEP) in HIV-uninfected women. HPTN 084 has currently enrolled more than 3,200 women who are between the ages of 18 to 45 years old in sub-Saharan Africa who are at risk for acquiring HIV. HPTN 084 is important as PrEP agents are needed that do not depend on daily or near-daily pill-taking. The development of alternative agents for PrEP, and/or more adherence-friendly schedules for currently available agents, could increase prevention choices and increase acceptability. Long-acting injectable agents have the potential to prevent HIV acquisition without relying on adherence to a daily oral regimen. Below is an image of the HPTN 084 study design.